Abstract
Background: Mogamulizumab-kpkc (mogamulizumab) is an anti-CCR4 monoclonal antibody approved for relapsed/refractory mycosis fungoides (MF) or Sézary syndrome (SS). In the Phase III MAVORIC trial comparing mogamulizumab to vorinostat, mogamulizumab-associated rash (MAR) was reported in 25% of patients (pts) and was more common in responders than in non-responders. Since then, several small case series have suggested that MAR is associated with response to the drug and prolonged remission.
Methods: We collected retrospective data from 8 academic centers on pts diagnosed with CTCL as defined by 2018 WHO-EORTC classification, who received at least one dose of mogamulizumab from 1/1/2000 to 1/31/2022. Primary endpoints were PFS and overall survival (OS) as calculated by Kaplan-Meier, compared with log rank test. Secondary endpoint was response to mogamulizumab in accordance with ISCL/EORTC 2011 criteria. MAR was graded with NCI CTCAE version 5.0. Categorical variables were compared between groups using Fisher test. Quantitative variables are presented with means and standard deviation or median and interquartile range (IQR). Univariate analysis was performed to calculate the hazard ratios (HR) for PFS.
Results: A total of 159 pts were included in analysis: 80 (50%) pts had a diagnosis of MF, 67 (42%) had SS, and 11 (7%) had other CTCLs. Median age was 70 (IQR 59.5-76), 65 (41%) were female, 124 (78%) were non-Hispanic Caucasian, 22 (14%) were Black. Median of prior lines of therapy was 3 (0-14). ECOG was 0-1 in 80% of pts at start of mogamulizumab. Majority of pts (70%, n=111) had advanced stage disease (Stage III or IV). Extranodal disease other than skin was present in 111 (70%) and LDH was elevated in 80 (50%) of pts.
MAR occurred in 72 (45%) pts. Majority of rash was grade 1-2 (76%, n=55) but 14% (n=10) developed grade 3 rash. Median time to onset was 3.7 months (mo) (IQR 2.1, 7.6) and median time to resolution was 4.3 mo (IQR 1.4, 7.5). Skin biopsy was obtained in 51 of 72 pts who developed MAR. Histologic patterns ranged from spongiotic/psoriasiform (40%, n=29), interface (19%, n=14), lichenoid (28%, n=20), granulomatous (7%, n=5) or a combination of patterns. Location of rash occurred in extremities (78%, n=56), trunk (69%, n=50), face/scalp (39%, n=28), buttocks/groin (8%, n=6) or combination of places. Of the pts who developed MAR, 24 (33%) were treated with topical steroids, 13 (18%) with systemic steroids, 16 (42%) with both topical and systemic steroids and 12 (17%) with other therapies, while 4 (6%) did not receive any treatment.
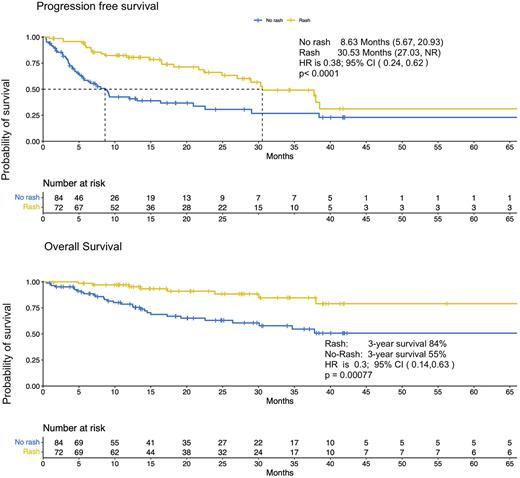
Median follow up was 20.9 mo, IQR: (12.0, 38.4). Median PFS was 30.5 mo (95% CI: 27.0 mo to not reached) for pts who developed MAR vs. 8.6 mo (95% CI: 5.7-20.9) for those who did not (HR=0.38, 95% CI: 0.24-0.62, p<0.0001). Three-year OS for pts with MAR vs. those without was 84% vs 55% (HR 0.3, 95% CI: 0.14-0.63, p=0.0008).
Complete remission (CR) was higher in pts with MAR (64%; n=46) than those who did not develop MAR (26%; n=23). Overall response rate (ORR) was also higher in those with MAR vs those without, 85% (n=61) vs. 54% (n=47), p<0.0001. Odds ratio of CR in pts with MAR was 4.64 (95% CI: 2.37-10.25), p<0.0001.
CR and partial remission (PR) in the blood were the most predictive of longer PFS on univariate analysis with HR of 0.05 (95% CI: 0.02, 0.14, p<0.001) and 0.07 (95% CI: 0.03, 0.20, p<0.001), respectively. Advanced stage CTCL at the start of mogamulizumab was also associated with improved PFS compared with early stage, HR 0.61 (95% CI: 0.37, 1.00), p=0.05.
At last follow-up, mogamulizumab had been discontinued in 114 pts due to rash (n=38), immune event (n=2), progression (n=56) or other adverse outcome (n=22). Mogamulizumab was held in 40 pts with drug rash. Of the pts who permanently discontinued mogamulizumab due to rash, 16 of 38 pts (42%) remain off systemic therapy at last follow up with ongoing response; median time off of systemic therapy for these pts was 7.8 mo (IQR: 6.1, 16.5).
Conclusions: The development of MAR in patients with CTCL is associated with higher rates of CR and ORR and significantly longer PFS and OS. CR/PR response in blood were associated with improved PFS. Additionally, 40% of patients who developed MAR and discontinued mogamulizumab had ongoing responses without additional systemic therapy. Rash may be a surrogate marker of favorable outcome with mogamulizumab.
Disclosures
Hu:Genentech/Roche: Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Atrash:Takeda: Honoraria; Sanofi: Honoraria, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; GSK: Honoraria, Research Funding; Celgene: Honoraria, Speakers Bureau; Amgen: Research Funding. Barta:Affimed: Consultancy; Daiichi Sankyo: Consultancy; Kyowa Kirin: Consultancy, Honoraria; Seagen: Honoraria; Janssen: Other: Independent Data Monitoring Committee member; Acrotech: Honoraria. Sokol:Kyowa-Kirin: Honoraria, Research Funding; Dren Bio: Consultancy. Moyo:Seattle Genetics: Consultancy. Ghosh:Adaptive Biotech: Consultancy; Genmab: Consultancy; Loxo Oncology: Consultancy; Novartis: Consultancy; Roche/Genentech: Consultancy, Research Funding; Karyopharm: Consultancy; Incyte: Consultancy; Beigene: Consultancy; Gilead: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; TG Therapeautics: Consultancy, Research Funding; AbbVie: Research Funding; ADC Therapeautics: Consultancy; Epizyme: Speakers Bureau; Morphosys: Research Funding; Seagen: Consultancy; Levine Cancer Institute: Current Employment, Honoraria, Research Funding. Shinohara:Calbaletta Bio: Research Funding; Elorac: Research Funding; NCCN T cell Lymphoma Panel: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal